Vision is our most important sense, which makes visual impairments a heavy burden for affected patients. AmblyoCare offers a variety of individually customizable stimulation methods, which provide a wide range of possibilities for the diagnosis and therapy in the area of early support for infants and toddlers (ESIT), amblyopia treatment and other visual impairments. Although the optimal rehabilitation of residual vision is achieved by frequent therapy in early age, AmblyoCare does not only target infants, but also adults with impaired vision.
The target audiences of AmblyoCare are ophthalmologists, orthoptists, ESIT specialists, pediatrists, neurologists, medical specialists as well as affected people (application only under professional guidance!).
AmblyoCare offers the following stimulation methods:
- Visual attention test
- Moving and static bars (optokinetic nystagmus)
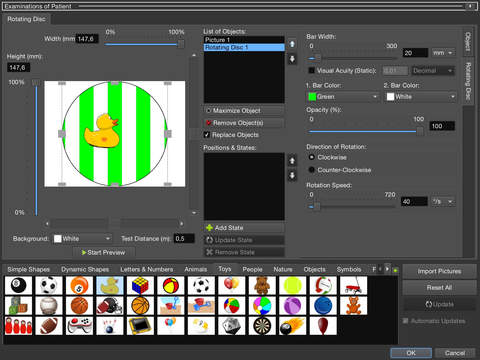
- Rotating disc (eccentric fixation)
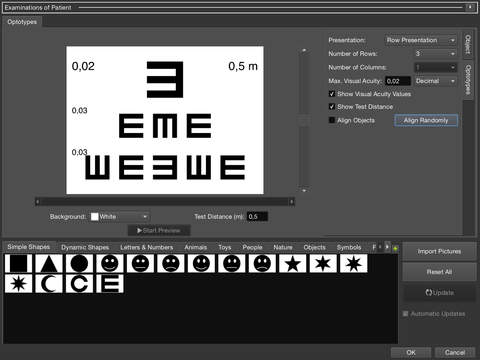
- Visual acuity test with optotypes (tumbling E, Landolt C, …)
- Smooth pursuit movements
- Saccades
- Preferential looking (only with suitable display hardware)
AmblyoCare is optimized for mobile devices with a screen size of at least 7 inches. In order to use AmblyoCare for more than 10 minutes and to start stimulations in full screen mode, a license must be purchased via In-App billing. The license is offered in the form of a one month subscription with a price of 49.99 USD which automatically renews every month unless auto-renew is turned off at least 24-hours before the end of the current subscription period. When you subscribe for the first time, you have a free trial period of 7 days to try out AmblyoCare. Any unused portion of the free trial period will be forfeited when you purchase a subscription. Payment will be charged to your iTunes Account at confirmation of purchase. Your account will be charged for renewal within 24-hours prior to the end of the current period for the following month at a price of 49.99 USD. Subscriptions may be managed by the user and auto-renewal may be turned off by going to your Account Settings after purchase. No cancellation of the current subscription is allowed during active subscription period.
AmblyoCare is developed in the frame of the research project SEE-KID/CEVD by the RISC Software GmbH, a company owned by the Johannes Kepler University Linz and the Upper Austrian Research GmbH. The project is supported by grants of the Upper Austrian local government, the convent hospital "Barmherzige Brüder" Linz and the general hospital of Linz (AKH Linz).
AmblyoCare is registered in the Austrian register of medical devices as an active medical device of class I and is intended for temporary use with a patient. Using AmblyoCare without medical supervision or not corresponding to advices of medical professionals is prohibited! AmblyoCare has the CE marking, which means that it is in compliance with the European Medical Device Directive 93/42/EEC and the Austrian medical-device-law BGBl. 657/1996 as amended in its current form. The software as well as the user manual are available in German and English and may only be used in those European countries where one or both of these languages is defined as an official language.
Please read the user manual (available at www.amblyocare.at) together with the included safety instructions carefully before using AmblyoCare and follow the directions concerning the correct usage of AmblyoCare. The use of AmblyoCare and the user manual is based on the currently applicable general terms of delivery of the RISC Software GmbH, which can be accessed at www.risc-software.at/en/agb.